Assessment of elamipretide in Barth syndrome - TAZPOWER Clinical Trial
October 17, 2019 Press Release
Stealth BioTherapeutics announced the presentation of new data from the open-label extension portion of the Phase 2/3 TAZPOWER study evaluating elamipretide in patients with Barth syndrome. The findings, presented at the American Society of Human Genetics (ASHG) 2019 Annual Meeting in Houston, Texas, showed that treatment with elamipretide resulted in a 27% increase in average cardiac stroke volume, or the amount of blood pumped by the heart's left ventricle per contraction, from the trial baseline (40.8 mL) to week 36 (51.8 mL) of the open-label extension. Left-ventricular stroke volume is one of the primary determinants of cardiac output, or the volume of blood pumped by the heart, which is an important indicator of how efficiently the heart can meet the body's demands for perfusion to various organs. In unaffected adolescent boys, an average stroke volume of 85 mL would be expected.
"Based on the data presented showing an increase in stroke volume, treatment with elamipretide appears to have improved heart function, which might indicate cardiac remodeling," noted Dr. W. Reid Thompson, Associate Professor of Pediatrics at the Johns Hopkins University School of Medicine. "Most patients with Barth syndrome have underlying heart disease, so a cardiac effect would be an important outcome in this setting that warrants further investigation."
Please click here for the full press release.
The following is a summary of a live presentation offered through collaboration with the Barth Syndrome Foundation (“BSF”) to the Barth syndrome patient and family community March 21, 2018. Stealth BioTherapeutics’ CEO Reenie McCarthy and Chief Clinical Development Officer Jim Carr, Pharm.D., presented an update on Stealth’s clinical trials in Barth syndrome and primary mitochondrial myopathy and answered questions from the community.
This summary and the content within is provided for reference purposes and for the intended audience only. Such reproductions and copies are authorized only when provided directly to the intended audience recipient by Stealth.
The BSF reminds our community that this webinar was intended to help inform and educate our families. It is in no way an endorsement of Stealth, or its investigational compound elamipretide. This summary and the content within does not intend to provide or substitute for medical advice. Please seek the advice of your physician about treatments which may be appropriate for you or your family member.
About Our Partnership with the Barth Community
Clinical trials are necessary to advance the development of experimental therapies toward potential approval. Extensive experimentation in laboratory experiments and in animals is prudent before conducting clinical trials, and for that, we are indebted to Bill Pu, Nathan Alder, Hazel Szeto, and others on the BSF Scientific and Medical Advisory Board for their guidance and findings. Beyond that, however, we are tremendously, hugely, grateful to the Barth community. It is not easy, or fun, or convenient to participate in clinical trials. It entails travel, injections, assessments, and evaluations which can be highly disruptive and tiring for uncertain clinical benefit. It demands dedication, perseverance, and bravery.
We applaud the participants in our trials, and their families, who endure all this disruption in their day- to-day lives. We are humbled by the dedication of the investigators for our trials, who are undertaking this work on top of their usual, busy workload. We are grateful that the BSF and its scientific advisory board have supported this study and partnered with us in its execution. We truly hope this will help progress the understanding of this disease and bring us closer to a therapeutic intervention.
About Stealth BioTherapeutics
Founded in 2006, Stealth BioTherapeutics is a clinical-stage biopharmaceutical company developing investigational drugs for the treatment of diseases involving mitochondrial dysfunction. Our scientific focus is on the mitochondria and on the ways in which mitochondrial therapy can positively impact the lives of patients. We are committed to positively impacting the lives of patients with novel treatment options, and to offering healthcare professionals the opportunity to transform clinical practice and patient outcomes. Our compounds are being developed for mitochondrial diseases where there are no FDA-approved treatments, and where current approaches are often only palliative. Our lead candidate in development is elamipretide, an investigational drug which is formulated for both systemic and ophthalmic formulations, with the potential to modify disease through mitoprotection — the ability to improve mitochondrial function while decreasing oxidative stress.
The science underlying our lead investigational candidate is supported by independent, peer-reviewed publications and abstracts presented in internationally recognized scientific journals, congresses and symposia.
Currently, Stealth is conducting clinical trials for patients with primary mitochondrial myopathy, Barth syndrome, Leber’s hereditary optic neuropathy and dry age-related macular degeneration. The Barth syndrome trial is named TAZPOWER.
Elamipretide and Mitochondrial Energetics
Click on image to view full screen
Mitochondria are found in nearly every cell in the body and produce about 90% of the energy (ATP) essential for human life. Mitochondria have two membranes, an outer and inner mitochondrial membrane. ATP is produced by the electron transport chain (ETC) located within the curves, or cristae, of the inner mitochondrial membrane. Cardiolipin, a phospholipid found only in that inner membrane, is responsible for establishing the cristae structure. Elamipretide targets and binds to cardiolipin,
stabilizing it under oxidative stress. More recent experiments have demonstrated that elamipretide also binds to monolysocardiolipin, which may have a similar stabilizing effect.
Click on image to view full size
The cristae curvature of the inner mitochondrial membrane (IMM) is important structurally, because it keeps the complexes of the ETC in optimal close proximity to one another. In dysfunctional mitochondria, high levels of oxidative stress can cause peroxidation of cardiolipin, which alters its normal structure. This in turns leads to degradation of cristae architecture, which in turn leads to displacement of the complexes of the ETC and further impairment of ETC function.
Cardiolipin and the Mitochondrial Membrane
As stated previously, elamipretide targets and binds reversibly to cardiolipin in the inner mitochondrial membrane, stabilizing it under oxidative stress. This effect was illustrated during an experiment using guinea pig cardiomyocytes, or heart cells. The heart cells had healthier membrane potential and cell viability during oxidative stress when treated with elamipretide as compared to placebo. Oxidative stress damages cardiolipin, causing mitochondrial dysfunction and triggering apoptosis, or cell death.
Although this experiment was done ex-vivo (not in a live animal) using healthy guinea pig cardiomyocytes, and so may not be predictive of humans with genetic mitochondrial disease, it does help illustrate the protective role elamipretide appears to have for mitochondria undergoing oxidative stress.
Cardiolipin in healthy heart and skeletal muscle mitochondria, or 18:2:4 cardiolipin, has 4 fatty acyl chains which give it a conical structure that creates the curved backbone of the cristae of the inner mitochondrial membrane. As discussed above, the curves of the cristae help keep the ETC complexes close together, optimizing ATP creation and minimizing electron leak and associated reactive oxygen species generation.
Unhealthy mitochondria have abnormal cardiolipin, often because the cardiolipin has been peroxidized, or degraded, by oxidative stress. This alters its conical structure and leads to abnormal cristae structure, which then allows electron transport chain complexes to drift apart, increasing electron leak and causing even more oxidative stress.
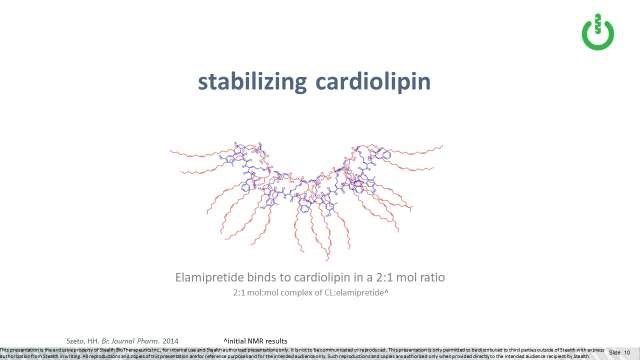
Data suggests that one molecule of elamipretide can bind to two cardiolipin molecules (Szeto, 2014). Because of its unique structure, elamipretide interacts with or binds to cardiolipin in two places, at the top (or head groups) and the tails (or fatty acid chains) of the molecule. We believe that elamipretide’s interaction with cardiolipin helps stabilize cardiolipin from the damaging effects of oxidative stress, thereby preventing or lessening further mitochondrial membrane dysfunction and ultimately cell death.
In Barth syndrome, the cardiolipin deficiency is primarily due to a genetic mutation resulting in abnormal cardiolipin composition. The genetic mutation impacts tafazzin, a cardiolipin remodeling enzyme, resulting in a form of cardioliopin called monolyslcardiolipin, or MLCL. MLCL differs from typical cardiolipin in that it has only three “tails”, whereas typical cardiolipin has four “tails” as discussed above. Importantly, this change alters the function of and reduces energy production in the mitochondria.
Click on image to view full size
Animal Studies Evaluating Elamipretide
High-energy organ systems are more dependent on mitochondrial energy production. In fact, the heart alone produces about 6 kg of ATP per day! Consequently, mitochondrial dysfunction disproportionately impacts high-energy organ systems. In patients with Barth syndrome, skeletal muscle function and cardiac function are often compromised, causing weakness, fatigue and cardiomyopathy. Similarly, in patients with other primary mitochondrial diseases, skeletal muscle, heart and eye function may be impaired, resulting in muscle weakness, exercise intolerance, cardiomyopathy, vision loss, etc. Normal mitochondrial structure and function are important for these organ systems to function properly.
Preclinical (animal) disease models have shown that treatment with elamipretide restores mitochondrial structure and function, with associated increased ATP production and decreased ROS generation to normal or near normal levels, and decreased inflammation, fibrosis and cell death (Szeto et al, 2015; Szeto and Birk, 2014). Treatment with elamipretide has also been found to improve organ function in animal models of aging skeletal muscle, heart failure, acute kidney injury, neurodegenerative diseases, and diseases of the eye. However, additional studies in humans are still necessary to assess the potential efficacy of elamipretide in individuals with these diseases.
Click on image to view full size
The Journey of Rare Disease Drug Development
The process of developing therapeutics for rare diseases involves multiple stages, from discovering potentially therapeutic compounds, to testing them for safety and efficacy in cells, tissues and animals, to testing them for safety in healthy volunteers, and then to testing them for safety and efficacy in people with disease. This process typically takes a decade or longer.
Click on image to view full size
The process begins by screening many molecules to look for key physical and chemical characteristics. Testing is subsequently performed in animals to rule out obvious toxicities as well as to understand if the chosen molecules display activity in various disease models. Of note, from the time that a molecule is chosen to undergo pre-clinical testing, there is only about a 5-10% likelihood that the drug will be successfully commercialized.
Assuming no obvious toxicity is identified and the molecule seems to have activity in disease models, the molecule advances to clinical testing in Phase 1 clinical trials, which are typically conducted in volunteers without disease to evaluate safety in humans. From Phase 1, the molecule advances to Phase 2 clinical trials, typically conducted in individuals with a disease and designed to further evaluate safety and to assess efficacy (favorable impact on signs and symptoms of disease). If the drug appears safe and effective, confirmatory testing is performed in Phase 3 clinical trials. Generally speaking, outside the rare disease setting, this process will involve over 1,000 human subjects. If the drug is confirmed to be safe and effective in Phase 3, the sponsor (i.e. the pharmaceutical company) will file for an approval with the FDA, which typically takes about a year. Combined, it’s not uncommon for this process to take 10 years or more.
For ultra-rare diseases like Barth, for which there are no approved therapies and the number of affected persons is small, it may be challenging to conduct multiple clinical trials from a recruitment perspective. For this reason, if data from the ongoing TAZPOWER trial is suggestive of clinical benefit, Stealth may discuss with the FDA the potential to seek approval on the basis of this data.
Findings from Clinical Trials in Primary Mitochondrial Myopathy (PMM)
MMPOWER, Stealth’s first clinical trial enrolling patients with PMM, was a randomized, double-blind placebo-controlled trial testing the safety, tolerability and efficacy of three different doses of elamipretide administered once daily intravenously over five days to 36 patients (age 16-65) with PMM. Treatment with elamipretide appeared to be well tolerated, and no serious adverse events were observed. Patients receiving the highest dose of elamipretide demonstrated a 44-meter placebo- adjusted improvement in the six-minute-walk test (6MWT), an assessment measuring how far they could walk in six minutes. This reached nominal significance and supported further study of elamipretide in this patient population.
MMPOWER-2, Stealth’s Phase 2 clinical trial for individuals with PMM, was a randomized, double-blind, placebo-controlled trial to evaluate the safety, tolerability and efficacy of elamipretide administered once daily subcutaneously to 30 subjects (age 16-65) with PMM. This was a 12-week crossover trial, meaning that participants were randomized to receive injections of either elamipretide or placebo for an initial 4-week period, after which they received no treatment during a 4-week “wash-out” period, before crossing over to receive the opposite injection during the last 4-week period. The TAZPOWER study for individuals with Barth syndrome has a similar crossover trial design, but the duration of each treatment period is 12 weeks.
The purpose of MMPOWER-2 was to further assess safety as well as evidence of efficacy across multiple endpoints to support a Phase 3 trial for individuals with PMM. Top-line results from MMPOWER-2 showed improvements in many endpoints, including the following endpoints:
- 6MWT: subjects receiving elamipretide walked an average 20 meters further on the 6MWT than those receiving placebo, although this difference did not achieve statistical significance.
- PMMSA Total Fatigue: The PMMSA (Primary Mitochondrial Myopathy Symptom Assessment) is a patient-reported outcome tool developed by Stealth pursuant to which individuals with PMM report their fatigue, muscle weakness and other bothersome symptoms on a score of 1 (least severe) to 4 (most severe) per symptom. While subjects were receiving elamipretide, they reported significant improvements in fatigue, which were highly statistically significant versus how they rated their feeling of fatigue while they received placebo.
-
NeuroQoL fatigue scale: The NeuroQoL fatigue scale was developed by the National Institutes of Health and has been utilized to measure fatigue in many trials for neuromuscular diseases. In MMPOWER-2, subjects receiving elamipretide showed a statistically significant and clinically meaningful improvement in fatigue on this scale.
-
PMMSA Most Bothersome Symptom: When subjects enrolled in MMPOWER-2, they were asked to identify which of the PMMSA-identified symptoms was most bothersome to them personally. In addition to fatigue and muscle weakness, these symptoms included headache, gastrointestinal symptoms, balance, vision, etc. While subjects were receiving elamipretide they experienced a statistically significant improvement in their individual “most bothersome symptom” assessed by the PMMSA.
-
MMPOWER-3 is the current, Phase 3 trial being conducted for individuals who completed REPOWER, an observational study in PMM. During MMPOWER-3, we will use many of the same endpoints that were used in MMPOWER-2.
Safety, Tolerability and Administration of Elamipretide
Elamipretide has been administered either by IV infusion or subcutaneous injection to over 500 healthy volunteers and patients. Treatment with elamipretide has generally appeared to be safe and well- tolerated, with no serious adverse events relative to placebo controls across the studies conducted to date. However, full characterization of elamipretide’s safety profile is ongoing and must continue to be assessed carefully before any definitive statements can be made about the safety profile of the investigational product.
Elamipretide and its placebo control are dosed subcutaneously (self-injected daily) in both the TAZPOWER and MMPOWER-3 trials. The most common side effects observed with subcutaneous administration are transient injection-site reactions which have typically been characterized as mild in nature. Most of the TAZPOWER trial participants have reported injection-site reactions during the first treatment period, suggesting that these reactions occur with both elamipretide and placebo for individuals with Barth who are participating in the TAZPOWER study.
Preclinical Studies in Barth Syndrome
Stealth Biotherapeutics is grateful to the BSF and its scientific and medical advisory board for advocating for a study examining the effect of elamipretide on individuals with Barth syndrome, and for its partnership with respect to related preclinical work conducted by us as well as by independent academic researchers.
Click on image to view full size
As mentioned, elamipretide binds to typical cardiolipin, also called 18:2:4 cardiolipin, in a ratio of two cardiolipin molecules to one elamipretide molecules, stabilizing it under oxidative stress. As discussed above, it appears that elamipretide binds to both the top (or head groups) and the tails (or fatty acid chains) of the cardiolipin molecule. We think this may be meaningful in the context of Barth, in which the “tail” composition of MLCL is what differs from typical cardiolipin, in that MLCL has three versus the typical four “tails”. The top or head group structure is the same as between MLCL and cardiolipin, so we would expect elamipretide may still bind there.
Cell-based experiments in lipid bilayer modeling systems also suggest that elamipretide binds to MLCL in the same ratio as it binds to typical cardiolipin (Alder & Szeto, et.al.). This provides preclinical rationale for the hypothesis that elamipretide may similarly bind to monolysocardiolipin in individuals with Barth.
In another ex-vivo experiment conducted by Dr. Bill Pu using cardiomyocytes, or heart cells, derived from Barth individuals as well as normal heart cells, elamipretide improved mitochondrial function in Barth-derived cells (Cardiomyopathy by Medical Therapy in a Mouse Model of Barth syndrome, presented at 2016 BSF Conference, Clearwater Beach, FL, July 22, 2016). In this experiment, Barth- derived cardiomyocytes were exerting more effort at rest than normal heart cells, suggesting they need to work harder to produce the minimal energy needed in a resting state. Conversely, the Barth-derived cardiomyocytes were unable to expend significantly more effort during maximal exertion, suggesting they do not have the spare capacity to ratchet energy production on demand. Presumably then, dysfunctional mitochondria require far more “effort” to produce ATP, even when at rest. When treated with elamipretide, the Barth-derived cardiomyocytes behaved closer to normal.
Although this is an experiment in Barth-derived cell lines and not in a living animal, these and other preclinical studies provided scientific support for elamipretide as a therapeutic candidate for Barth.
Click on image to view full size
TAZPOWER
The design of the TAZPOWER trial is similar to that described above for MMPOWER-2. TAZPOWER is a double-blind, placebo-controlled, randomized crossover trial. Individuals participating in the trial receive either elamipretide or placebo for a treatment period of 3 months, then are “crossed-over” to the opposite treatment after a washout period. Given that Barth syndrome is very rare, only 12 individuals are enrolled; the cross-over trial design provides more statistical power, which is particularly important with so few subjects enrolled. Multiple endpoints are being evaluated during this trial, most of which were chosen based on feedback from clinicians and Barth individuals about the functional deficits (i.e. exercise intolerance, weakness) and symptoms experienced by individuals with Barth. These include the BarTH Syndrome Symptom Assessment (BTHS-SA), a novel patient-reported outcome assessment tool which was developed by Stealth based on information gathered during interviews with people who live with Barth syndrome. Once subjects complete the trial, they have the option to continue to receive the drug during an open-label extension period.
Patient Voices in Rare Diseases
The FDA has repeatedly signaled the importance of incorporating the “patient voice” in drug development. That is even more critical in rare diseases. For example, the 21st Century Cures Act requires sponsors to include the patient perspective. PDUFA, the prescription drug user fee act, specifically encourages the use of patient reported outcomes. These regulatory initiatives underscore the importance of patient reported outcome measures such as the BTHS-SA we developed for the TAZPOWER trial.
The voice of rare disease individuals and advocacy is critical in educating the FDA. In a very small clinical trial like TAZPOWER, it may be challenging to see compelling clinical data that reaches statistical significance. The patient voice may be particularly critical in this setting. Stealth intends to remain actively engaged with both the BSF community and the FDA with the goal of bringing therapeutic options to patients who suffer from Barth syndrome as quickly as possible.
Community Q&A
Many boys and young men with BTHS have had a heart transplant and currently are excluded from the elamipretide trials. Please explain why and if there will be an opportunity for these individuals to participate in the future.
Individuals who have had a heart transplant were excluded in an effort to minimize any confounding variables that would impact the results of the trial. Aggressive attempts are made to minimize the variables so that endpoints (measurable outcomes) of the study can be confidently associated with the investigational drug and not be influenced by some other variable. In this case, while people who have had BTHS and a heart transplant were not eligible for TAZPOWER, if the drug were approved, then those individuals would have access to the drug if their prescribing physician deemed it appropriate.
What does Fast Track Status (granted to Stealth for elamipretide in the study of BTHS by the FDA in 2017) and open-label extension period mean?
Fast track status granted by the FDA provides a facilitated pathway between the company and the FDA, and simply implies a higher level of engagement and partnership due to the importance of the clinical trial. It does not grant broader or quicker access to the investigational drug for patients.
Open-label extension (OLE) for patients with rare diseases is important as it allows patients to continue therapy until the drug is approved if there is benefit. OLE is available for patients in the TAZPOWER trial until the drug is approved for Barth Syndrome or until Stealth stops development efforts in this disease. OLE also provides an opportunity for ongoing safety data to be gathered.
If cardiolipin is deficient in people with BTHS, how does elamipretide work (since elamipretide binds to cardiolipin)?
Ex-vivo preclinical work has demonstrated that the binding ratio of elamipretide to monolyscardiolipin is the same as normal cardiolipin, and has also shown improvement of function in Barth-derived cardiomyocytes. This work supports the hypothesis that elamipretide may be able to stabilize the inner mitochondrial membrane in individuals with BTHS despite a cardiolipin deficiency. However, we will need to assess the data from the ongoing TAZPOWER trial to ascertain whether the clinical data is supportive.
Why were children under the age of 12 not allowed to participate in the TAZPOWER study, and will these children ever have access to the drug?
Historically, the FDA has encouraged sponsors to demonstrate the safety and efficacy of new investigational products such as elamipretide in adults before allowing access to pediatric patients. TAZPOWER was limited to individuals over the age of 12 for this reason. However, Stealth is keenly aware of the disease burden Barth syndrome presents to young children, and is committed to developing therapeutics for children with Barth if elamipretide is shown to be efficacious in adults with Barth. Stealth is accordingly evaluating the potential for a Barth syndrome clinical trial in children, including assessing age and dosing limitations, which would likely be on a mg/kg basis rather than the standard subcutaneous dose currently used in TAZPOWER and MMPOWER-3.
Of Stealth’s clinical trials, which is most promising and most likely to bring a drug to approval?
While Stealth has conducted the most clinical work in mitochondrial myopathy, it is possible that TAZPOWER would be the first trial to bring elamipretide to approval.
Does elamipretide have an effect on athletes and performance? Why was the 6MWT (six-minute walk test) chosen as a primary endpoint in the TAZPOWER and MMPOWER trials?
Elamipretide has no effect on normal mitochondria. In mouse models, a young, healthy mouse has no benefit when dosed with elamipretide, so we would not expect, for example, that athletes or other healthy people would experience benefit from elamipretide. However, in an old mouse with mitochondrial dysfunction, elamipretide restores mitochondrial function to near normal levels.
Clinical trials should have functional and subjective endpoints that allow investigators to measure benefit in a consistent way across all patients. This is to provide regulators with confidence as to whether the clinical outcomes, such as fatigue and skeletal muscle function, improve in a statistically significant manner.
TAZPOWER and the MMPOWER trials include both subjective tests, such as the NeuroQoL and the PMMSA/BTHS-SA, and functional tests, such as the 6MWT. The 6MWT, which measures skeletal muscle function by assessing how far an individual can walk in 6 minutes, is an endpoint that is well-recognized by regulators in the US and in Europe.
Will dose be adjusted based on weight during the trial or the OLE period? Is weight is a factor in how well the drug might work, or if once it’s in the body a standard dose should work equally for people of such varying weight? Some 12 year olds might barely make the weight requirement and others may weigh much more.
Although Stealth investigators observed evidence of a dose response in one of our trials, after a certain degree of drug exposure is achieved we don’t believe that there are additional benefits from pushing the exposure even higher. Therefore, despite the recognition that there could be considerably higher drug exposure in one subject versus another, we don’t believe that this will result in differential effectiveness. Generally speaking, once we have reached a therapeutic dose (which we believe is the 40mg dose we are testing in TAZPOWER), there doesn’t appear to be a strong correlation between even higher concentrations and effects with elamipretide.
Could it be possible that the drug could take longer in some Barth subjects than others to prove beneficial?
Conceptually, it could take longer to see a response in one subject versus another. For example, there could be differences in the amount of oxidative stress that exists in the mitochondria between one person and another. Also, it may depend on the effect that one is looking for. For example, it may take more time to see an improvement in exercise performance versus an improvement in symptoms.
Does age make a difference in effectiveness of elamipretide? For example, the older a Barth subject is, the less effective the drug might be in helping with the fatigue. However, the presentation spoke to the effect on “aging animals”. Were those “normal” aging animals, so elamipretide helps with “normal aging” but not the impact of aging on individuals who also have Barth syndrome?
Age, per se, should not impact the effectiveness of the drug. Instead, what has been observed in animal studies, as well as in some human studies, is that more dysfunctional mitochondria seem to respond better to the drug. In other words, the more dysfunction that was present the better the drug seemed to work. To your point, the reason we believe we see signs of benefit in “normal aging” is because mitochondrial dysfunction is associated with “normal aging”; whereas with Barth, the mitochondrial dysfunction typically exists from birth.
Aging can lead to a reduction in mitochondrial performance so it’s possible that the drug could have additional benefits as a Barth individual continues to age, but we would need further data to be able to assess this.
In many drugs, the specific mutation makes a difference. Is that the case with Barth syndrome and elamipretide?
Very good point. For example, some individuals with Barth Syndrome might have more of an ability to make tafazzin. It is possible these individuals would either respond better or worse to the drug. These are subtleties we were not able to ascertain from the preclinical experiments we conducted.
Is it possible to improve in the 6MWT by 44 meters or more and yet the person doesn’t actually feel better? Does staying on the drug longer potentially help more?
Yes, it’s possible. The 6MWT describes the impact on physical functioning, but does not represent changes in symptoms. The hope is that the drug can improve both physical functioning and symptoms. Also, one would hope that an improvement in physical functioning would lead to an ability to better perform activities associating with daily living, thereby improving quality of life. It is also possible that longer treatment with elamipretide could help, in that improvement of mitochondrial function may in turn lead to improvement in end organ function, but this is something we need further data to ascertain with respect to Barth.
When the Stealth team talks about weakness and fatigue, are they using those words interchangeably?
No, weakness refers to weakness in skeletal muscles. Fatigue is more related to feeling exhausted or extremely tired.
Since one of the elamipretide trials was specifically for heart failure, is there a possibility that there might be improvements in heart function for patients with Barth syndrome, even if there is not an improvement in the 6MWT?
Yes, there might be effects on the heart that are independent of the effects on exercise performance. We will need to evaluate the data once the trial is completed to understand this better.
Additional References
Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014 Apr;171(8):2029-50.
Szeto HH, Liu S, Soong Y, Birk AV. Improving mitochondrial bioenergetics under ischemic conditions increases warm ischemia tolerance in the kidney. Am J Physiol Renal Physiol. 2015 Jan 1;308(1):F11-21. Epub 2014 Oct 22.
Szeto HH, Birk AV. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther. 2014 Dec;96(6):672-83.
Sathappa M, Alder NN. The ionization properties of cardiolipin and its variants in model bilayers. Biochim Biophys Acta. 2016 Jun;1858(6):1362-72. Epub 2016 Mar 7.
Kimura T, Jennings W, Epand RM. Roles of specific lipid species in the cell and their molecular mechanism. Prog Lipid Res. 2016 Apr;62:75-92. Epub 2016 Feb 11.
For more information about Stealth BioTherapeutics, please visit www.StealthBT.com and www.clinicaltrials.gov.
Stealth BT would like to acknowledge the BSF (www.barthsyndrome.org) and the patients and families who suffer from Barth for their support and collaboration.