Mitochondria-Homing Drug Mitochonic Acid 5 Improves Barth Syndrome Myopathy in a Human-Induced Pluripotent Stem Cell Model and Barth Syndrome Drosophila Model
Authors: Yoshiyasu Tongu, Tomoko Kasahara, Tetsuro Matsuhashi, Yoshitsugu Oikawa, Ryota Akimoto, Yuhan Luo, Sayaka Sekine, Momoka Suzuki, Hitomi Kashiwagi, Shinichiro Kanno, Yoshikazu Tanaka, Kyohei Sato, Yusuke Okubo, Akihiko Muto, Hidetaka Tokuno, Chitose Suzuki, Chiharu Kawabe, Takamasa Ishikawa, Shun Watanabe, Koichi Kikuchi, Shun Itai, Takeya Sato, Takehiro Suzuki, Kazuhiro Igarashi, Shinji Fukuda, Tomoyoshi Soga, Kei Murayama, Erina Kuranaga, Takafumi Toyohara, Takaaki Abe
Journal: Federation of American Societies for Experimental Biology (Link to article)
Background:
Barth syndrome is a mitochondrial disorder caused by changes or variants in the TAFAZZIN DNA (What are genes and proteins? What is a mutation?). Changes to TAFAZZIN can cause it to malfunction, which leads to issues in mitochondria. Mitochondria are important energy factories present in nearly every cell of the body (learn more about mitochondria here!). Mitochondria in Barth syndrome have an abnormal shape and, as a result, do not produce enough energy causing issues with the heart, skeletal muscle and other organs.
Dr. Takaaki Abe, a physician scientist at Tohoku University in Sendai Japan, and his lab recently published a peer-reviewed paper showing how a drug called mitochonic acid 5 or MA-5 for short might improve some of the symptoms associated with Barth syndrome using cell and fruit fly models.
Findings:
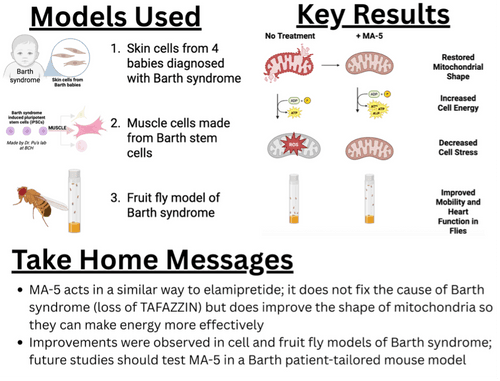
Dr. Abe’s research group has published many papers on MA-5, showing that it can improve mitochondrial function and energy production in different disease conditions including but not limited to Parkinson’s disease, Duchenne muscular dystrophy and inclusion body myositis. In this most recent paper, the research team tested their MA-5 compound on two different Barth syndrome cell lines as well as a fruit fly model of Barth syndrome (fun fact: one of the cell lines was generated in BSF Scientific and Medical Advisory Board [SMAB] member Bill Pu’s lab and the fruit fly model was developed by former SMAB Chair/current member Michael Schlame’s lab).
When MA-5 was added to cells or fed to fruit flies, they found that mitochondrial shape was improved, cells were able to make more energy (also called adenosine triphosphate or ATP), signs of cell stress were lower. In fruit flies, they found that, in addition to mitochondrial improvements, heart function was improved and the flies could climb better. These findings are similar to what has been observed with elamipretide—the drug does not cure the underlying condition but helps improve mitochondrial shape which in turn improves energy production. One MA-5 is different from elamipretide is how it is administered: MA-5 can be given by mouth, while elamipretide must be injected. The authors mention that a Phase 1 clinical trial was conducted in Japan—this trial was performed in healthy adults to look at how safe it is and what dose should be used. For this drug to move closer to clinical trials for the treatment of Barth syndrome, critical next steps would be to test the MA-5 compound in a patient-tailored mouse model like the one generated in Dr. Simon Conway’s laboratory. This will help determine whether MA-5 can improve heart and skeletal muscle function in a model that more closely approximates what humans with Barth syndrome experience.
Take-Home Message: This study provides early but promising data on a new compound that has the potential to improve mitochondrial shape and energy production in a pill that can be taken by mouth. Future experiments should evaluate how MA-5 performs in a mouse model of Barth syndrome



