A murine model of Barth syndrome with cardiac and skeletal muscle selective inactivation of tafazzin
Article title: A murine model of Barth syndrome with cardiac and skeletal muscle selective inactivation of tafazzin
Authors: Erika Yazawa, Erin M. Keating, Suya Wang, Mason E. Sweat, Qing Ma, Yang Xu, Michael Schlame, William T. Pu
Journal: Disease Models and Mechanisms
Background:
Barth syndrome is caused by a change (also called a variant or mutation) in a piece of DNA that produces the protein called TAFAZZIN. Changes in the TAFAZZIN DNA often mean that the protein does not function properly. In cells, the TAFAZZIN protein converts a fat (or lipid) called monolysocardiolipin (MLCL) into its mature form called cardiolipin (CL). Having the appropriate amounts of MLCL and CL is important for each cell’s mitochondria to make enough energy. In Barth syndrome, the TAFAZZIN protein cannot convert MLCL into CL, which causes the body to have too much MLCL and not enough CL. The buildup of MLCL and lack of CL prevents cells from producing enough energy to function properly. This is particularly problematic for the heart and skeletal muscles (e.g., biceps, quadriceps, hamstrings) because they need a ton of energy to function. The inability to produce enough energy weakens the heart and skeletal muscles, which causes the hallmark symptoms associated with Barth syndrome.
Scientists need appropriate animal models to identify and develop potential therapies. One way scientists can make an animal model is by replicating the mechanism that causes Barth syndrome. Since Barth syndrome is caused by a genetic change in TAFAZZIN DNA, scientists replicate that by using special techniques to remove or inactivate TAFAZZIN, typically in mice (this is called a knockout mouse). Removal of TAFAZZIN from all cells in a mouse produces traits that mimic Barth syndrome in humans (e.g., small body size, weakened heart and skeletal muscles). In mice, however, it is also associated with poor survival past the newborn stage. This represented a large obstacle as scientists were not able to easily test potential therapies in mice. This is because it was challenging to get enough surviving mice to test treatments or interventions. One way to circumvent this hurdle is to only remove TAFAZZIN from specific subset(s) of cells (e.g., cells in the heart or skeletal muscles) – this is called a conditional knockout model.
Findings:
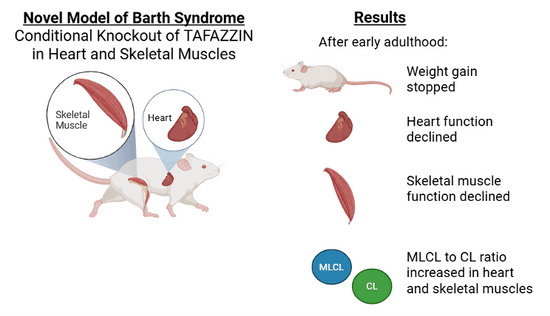
Scientists from Dr. Bill Pu’s lab removed TAFAZZIN from heart and skeletal muscle in mice, but not from other areas like the liver or bones. The scientists compared these conditional knockout mice to control mice that still had TAFAZZIN.
Mouse survival and size: Unlike mice who lack TAFAZZIN in all cells, the conditional knockout mice survived and grew similar to controls until early adulthood. After that, the conditional knockout mice stopped gaining weight while control mice continued to gain weight.
Heart function: Heart function began to decline in early adulthood for the conditional knockout mice. Eventually, the hearts weakened enough that they were not able to pump blood effectively, which means they developed systolic heart failure.
Skeletal muscle function and exercise capability: The scientists measured skeletal muscle function by testing endurance on a treadmill. The mice had similar treadmill endurance until early adulthood. After that, treadmill endurance declined for the conditional knockout mice.
Cardiolipin levels: Compared to controls, adult conditional knockout mice had a high amount of MLCL, a low amount of CL, and a high MLCL to CL ratio in heart muscle cells, which is similar to humans with Barth syndrome. Interestingly, in skeletal muscles, adult conditional knockout mice had a normal amount of MLCL, a slightly lower amount of CL, and a high MLCL to CL ratio, which is less severe than what is observed in humans with Barth syndrome.
Take-Home Message:
This conditional knockout mouse model is a new and important tool for the Barth syndrome research community because it recreates some of the most vital hallmarks of the disease but in a way that enough mice survive to early adulthood when drug interventions might typically be evaluated. In early adulthood, conditional knockout mice stopped gaining weight, heart function declined, exercise capability declined, and the MLCL to CL ratio increased in heart and skeletal muscles. These traits mimic Barth syndrome symptoms. One potential consequence is that not all symptoms characteristic of Barth syndrome may be present in the mouse model, which could prevent scientists from fully understanding how a drug functions. Nevertheless, this model is important because it can be used to screen and evaluate potential treatments in large numbers of mice that are equivalent in age to human children, teens, and adults.













